This blog is to discuss and share unique clinical experiences with the Surgeons and Gastroenterologist, for the better understanding of clinical problems.
Monday, 31 October 2011
Monday, 24 October 2011
Spigelian Hernia Repair in 80 Yr Male
Location:
Surat, Gujarat, India
Monday, 10 October 2011
Dr Rahul Naik's Blog on Gastroenterology, GI, Laparoscopic and Bariatric Surgery: Complications of PEG
Dr Rahul Naik's Blog on Gastroenterology, GI, Laparoscopic and Bariatric Surgery: Complications of PEG: http://www.tropicalgastro.com/articles/30/4/Complications-of-PEG--Prevention-and-Management.html
Labels:
Publication in Indexed Journal
Location:
Surat, Gujarat, India
Sunday, 9 October 2011
Sloughed off Caecum with Burst Liver Abscess in Amoebic Disease
Labels:
Caecal Perforation,
Liver Abscess
Location:
Surat, Gujarat, India
Saturday, 8 October 2011
Friday, 7 October 2011
Thursday, 6 October 2011
Encapsulating sclerosing peritonitis
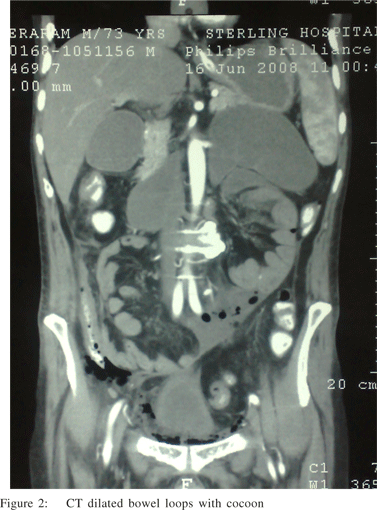
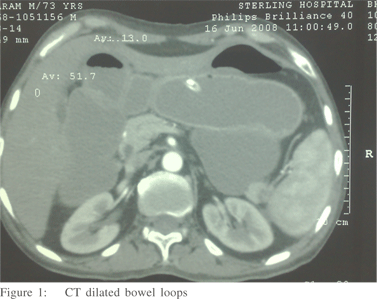
Sclerosing encapsulating peritonitis is a rare cause of small bowel obstruction, and can be classified as idiopathic or secondary (most importantly and most frequently due to chronic ambulatory peritoneal dialysis). The idiopathic form (also known as abdominal cocoon) was first described by Foo et al in 1978.[1,2] It affects mainly young females from tropical and subtropical regions, but adult case reports from temperate zones can be encountered in literature. It is characterized by a thick, fibrotic, cocoon-like membrane, partially or totally encasing the small bowel. Clinically, it presents with recurrent episodes of acute or sub acute small bowel obstruction, weight loss, nausea and anorexia, and at times with a palpable abdominal mass.[2] Most cases are diagnosed incidentally at laparotomy, as in the case presented, although a preoperative diagnosis is purported feasible by a combination of barium follow-through (concertina pattern or cauliflower sign and delayed transit of contrast medium) and computed tomography of the abdomen (small bowel loops congregated to the center of the abdomen encased by a soft-tissue density mantle).[3,4] However, preoperative diagnosis requires a high index of clinical suspicion. Surgery (membrane dissection and extensive adhesiolysis) is the treatment of choice, and there is usually no need for bowel loop resection, especially when a preoperative diagnosis is feasible. Resection of the bowel is unnecessary and it increases morbidity and mortality. Resection is indicated only if the bowel is non-viable. An excellent longterm postoperative prognosis is most of the times guaranteed.[5]
Case Presentation
A 70 year-old man was presented with 24-hour history of colicky abdominal pain and bilious vomiting. He reported to have 6 similar episodes, attributed to small bowel obstruction in the past 4 years, which required hospitalization and resolved with conservative treatment. He also admitted chronic constipation for the last 6 years, anorexia and 15 kg weight loss since his last admission. He had no surgical or other medical history. On examination, he was in distress, but afebrile and haemodynamically stable. His abdomen was distended but non- tender, with increased bowel sounds in pitch and frequency.
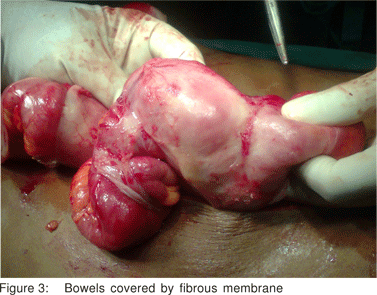
No palpable abdominal mass or organomegaly and no external hernias were present. Laboratory blood analyses were within normal limits. Plain abdominal X-ray showed few air-fluid levels centrally located, without free intraperitoneal gas. Ultrasound of the abdomen did not reveal any abnormalities. Contrastenhanced CT scan of abdomen (Figure 1,2), performed on the same day, confirmed the diagnosis of small bowel ileus without providing any diagnostic clues. The patient was admitted and ileus resolved by day 3 conservatively. Endoscopy of upper and lower gastrointestinal tract and biopsies from the duodenum and colon provided normal findings. Although symptoms of obstruction had abated, the history of multiple relapses, the patient’s complaints for poor quality of life and multiple admissions, as well as the undefined origin of the underlying pathology led to an exploratory laparotomy on day 7. On surgery, a fibrous capsule covering all the abdominal viscera was revealed, in which small bowel loops were encased, with the presence of interloop adhesions (Figure 3). The liver, stomach, appendix, right and left colon, as well as the sigmoid, were also covered and the greater omentum looked hypoplastic and encased in fibrous tissue. Incision of the thick membrane and extensive adhesiolysis of small bowel loops were performed without loop resection. Histology of the membrane showed thickened fibrocollagenous tissue without inflammation. A diagnosis of idiopathic sclerosing encapsulating peritonitis (abdominal cocoon) was established, due to intraoperative findings and by ruling-out any other condition explaining the patient’s pathology. Postoperatively, a forgotten small bowel follow-through performed elsewhere was brought to our attention by the patient, showing ileal loops bunched and confined in the lower abdomen and pelvis apparently due to adhesions. Postoperative recovery was uneventful and one year after the operation he is in good health.
Discussion
In the presented case, an intra-operative diagnosis of idiopathic sclerosing peritonitis was made in an adult male patient with a history of recurrent bouts of small bowel ileus. Pre-operative findings were inconclusive in his current admission. Although final surgical management would not have been modified, if results from imaging investigations during prior admissions were accessible in time, a pre-operative diagnosis could have been made possible. Idiopathic sclerosing encapsulating peritonitis or abdominal cocoon, although a rare cause of a common surgical emergency such as small bowel ileus, may be responsible, especially in cases with recurrent attacks of nonstrangulating obstruction in the same individual. A high index of clinical suspicion may be generated by the recurrent presentation of small bowel ileus combined with relevant imaging findings and lack of other etiologies. Preoperative diagnosis must be pursued, as it may prevent a “surprise” upon laparotomy and unnecessary procedures for the patient, such as bowel resection.
References
1. Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427–30.
2. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20:S43–55.
3. Deeb LS, Mourad FH, El-Zein YR, Uthman SM. Abdominal cocoon in a man: preoperative diagnosis and literature review. J Clin Gastroenterol. 1998;26:148–50.
4. Hur J, Kim KW, Park MS, Yu JS. Abdominal cocoon: preoperative diagnostic clues from radiologic imaging with pathologic correlation. AJR Am J Roentgenol. 2004;182:639–41.
5. Celicout B, Levard H, Hay J, Msika S, Fingerhut A, Pelissier E. Sclerosing encapsulating peritonitis: early and late results of surgical management in 32 cases. French Associations for Surgical Research. Dig Surg. 1998;15:697–702
Spontaneous rupture of spleen in dengue virus infection
Dengue virus infection is a common mosquito- borne disease found in tropic and subtropic regions of the world. It is transmitted to humans by the bite of Aedes aegypti, and rarely by Aedes albopictus mosquitoes. Dengue virus infection may be asymptomatic or lead to undifferentiated fever, dengue fever (DF) or dengue hemorrhagic fever (DHF). The increasingly widespread distribution and rising incidence of dengue virus
infection is related to the increased distribution of Aedes aegypti, and its increase in urban areas of Southeast Asia and due to air travel. All four dengue serotypes cause DF are characterized by sudden onset fever, headache, retro-orbital pain, general malaise, myalgias, leucopenia with lymphocytosis, thrombocytopenia, and mild elevation of liver enzymes. It may also cause rash, mild hemorrhagic manifestations; and rarely
severe hemorrhage leading to shock through blood loss. In a small percentage of dengue virus infections, a more severe form of disease, known as DHF, is characterized by acute fever associated with a hemorrhagic diathesis and a tendency to develop dengue shock syndrome (DSS). Treatment of both classic DF and DHF/DSS is symptomatic and supportive. There has been an apparent increase in the complications of
dengue infection seen among adults in our practice. We describe a case of spontaneous splenic rupture in a patient with DHF.
Case Report
A 25-year-old man was admitted with a 7-day history of fever, myalgia, and headache. He was febrile and anicteric. No pallor, rash or lymphadenopathy was evident, and a tourniquet test was negative. Mild hepatomegaly was present. The neck was supple with no neurologic deficit. Platelet count was 90,000/
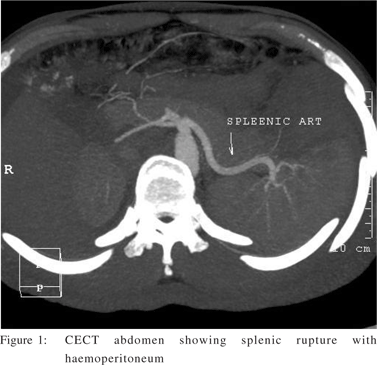
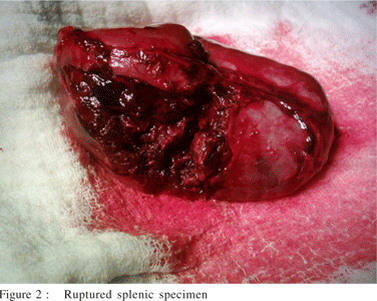
dL, and hematocrit was 33% before the admission which dropped to 35,000/dL and 30% at the admission. A peripheral blood smear did not show malarial parasites, and blood cultures were sterile. Dengue virus specific IgM antibodies were found to be positive. On day 2, the patient had sudden hypotension with abdominal pain and distension; hematocrit dropped to 15% and platelet count to 25,000/dL. Paracentesis yielded frankly hemorrhagic fluid. Contrast-enhanced computer tomography showed ascites and an organized, non enhancing collection over the posterosuperior aspect of spleen, suggestive of splenic rupture. After resuscitation with colloids and packed red cells, he was taken for emergency surgery. Exploratory laparotomy with splenectomy and lavage of peritoneal cavity was done. Spleen was ruptured at the lower
pole and at the hilum (Figure 2). Over the next few day he recovered uneventfully. His fever and platelets normalized in 10 days after the surgery. At present he is doing well.
Discussion
Often considered more common in children, DHF is now being seen more frequently in adults as a consequence of shifting patterns of infection and immunity. [4,5] Although the pathogenesis and pathophysiology of severe dengue infection remains incompletely understood, possible contributory factors to increased disease severity have been described.[5,6] Age, sex, race, pre-existing co-morbidities, and viral-specific features have been noted to play a role in disease outcomes.[3,7] The mainstay of treatment remains prompt fluid resuscitation to counteract massive plasma leakage. Timely and effective intravenous crystalloid replacement of plasma losses results in a favorable outcome in most cases.[8] In its severest form, dengue virus infection is associated with hemorrhagic complications, plasma leakage, shock, liver failure, and disseminated intravascular coagulopathy.
Dengue virus infections are rarely fatal in adults, although fatal infections do occur.[5,9] Bleeding, one of the major problems encountered in DHF, contributes to worsening morbidity. The pathogenesis of hemorrhage may be multifactorial and include vasculopathy, platelet deficiency and dysfunction, and blood coagulation defects.[10] The most common hemorrhagic manifestations are epistaxis, skin hemorrhages, and gastrointestinal hemorrhages. [5,11] Bleeding can occur in any organ. Spontaneous splenic ruptures are rare but lifethreatening complications of infectious diseases. The typical presentation is acute, but progressive forms are described.[12,13] The spleen, which frequently has congestion, bears subcapsular hematomas in 15% of DHF cases.[14]
Splenic rupture in patients with hemorrhagic dengue is uncommon but can happen spontaneously or as a result of trauma, which may be minor or unnoticed. There are only three previously reported cases of spleen rupture in patients with dengue fever: a 35-year-old white man with dengue fever who underwent splenectomy in French Polynesia and had a favorable clinical outcome;[15] a 23-year-old woman who lived in Venezuela, had severe illness and died after splenectomy with gramnegative sepsis and multiorgan failure;[16] and a 52-yearold woman with dengue fever who underwent splenectomy in Brazil and had a favorable clinical outcome.[17] In the first and third cases, the ruptured spleen developed in patients without the classical symptoms of DHF/DSS. We presume the splenic rupture in our patient was due to factors, such as the level of severity of DHF/DSS (grade IV) the presence of consumption coagulopathy and severe thrombocytopenia.
In conclusion, a case of spleen rupture may be misdiagnosed as shock syndrome seen in DHF/DSS. Physicians should be aware of the possibility of splenic rupture in areas where dengue infection is endemic. Early diagnosis and treatment are needed to avoid a fatal outcome.
References
1. Pongsumpun P, Patanarapelert K, Sriprom M, Varamit S, Tang IM. Infection risk to travelers going to dengue fever endemic regions. Southeast Asian J Trop Med Public Health. 2004;35:155–9.
2. World Health Organization. Dengue haemorrhagic fever. diagnosis, treatment prevention and control.2nd ed. Geneva: WHO press; 1997.
3. George R, Lum LCS. Clinical spectrum of dengue infection. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. New York: CAB International; 1997.p.89–114.
4. Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–93.
5. Pancharoen C, Kulwichit W, Tantawichien T, Thisyakorn U, Thisyakorn. Dengue infection: a global concern. J Med Assoc Thai. 2002;85:S25–33.
6. Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever.Curr Opin Infect Dis. 2006;19:429–36.
7. Nimmannitya S. Clinical spectrum and management of dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987;18:392–7.
8. Nhan NT, Phuong CXT, Kneen R, et al. Acute management of dengue shock syndrome. A randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis. 2001;32:204–12.
9. Nimmannitya S, Thisyakorn U, Hemsrichart V. Dengue hemorrhagic fever with unusual manifestations.Southeast Asian J Trop Med Public Health. 1987;18:398–406.
10. Halstead SB. Dengue: hemorrhagic aspects. Simin Hematol. 1982;19:116–31.
11. Kittigul L, Pitakarnjanakul P, Sujirarat D, Siripanichgon K. The differences of clinical manifestations and laboratory findings in children and adults with dengue virus infection. J Clin Virol. 2007;39:76–81.
12. Peters RM, Gordon LA. Nonsurgical treatment of splenic hemorrhage in an adult with infectious mononucleosis. Case report and review. Am J Med. 1986;80:123–5.
13. Zingman BS, Viner BL. Splenic complications in malaria: case report and review. Clin Infect Dis. 1993;16:223–32.
14. Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever. A study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61:500–10.
15. Imbert P, Sordet D, Hovette P, Touze JE. Spleen rupture in a patient with dengue fever. Trop Med Parasitol. 1993;44:327–8.
16. Redondo MC, Rios A, Cohen R, Ayala J, Martínez J, Arellano G, et al. Hemorrhagic dengue with spontaneous splenic rupture: case report and review. Clin Infect Dis. 1997;25:1262–3.
17. Miranda LE, Miranda SJ, Rolland M. Case report: spontaneous rupture of the spleen due to dengue fever. Braz J Infect Dis. 2003;7:423–5.
Subscribe to:
Posts (Atom)